new gene therapy platform for genetic muscle diseases.
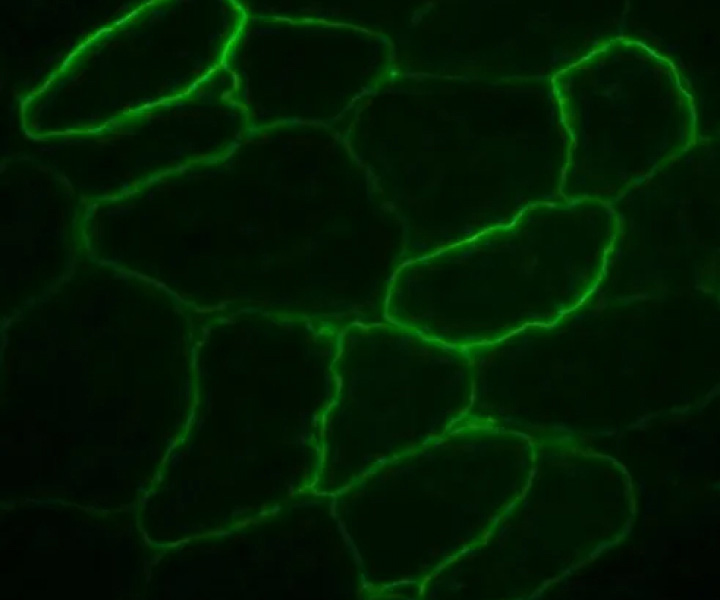 immunostaining-1
immunostaining-1Myosana is pioneering a targeted non-viral gene therapy platform for neuromuscular and cardiac genetic diseases. Our platform is designed to overcome the limitations of viral delivery, expanding the range of diseases that doctors can treat with gene therapy.
 iStock-1086669342
iStock-1086669342The Myosana platform has the potential to save lives and increase longevity, as well as improve quality of life for patients and their families who now face significant unmet needs in genetic disease therapy.
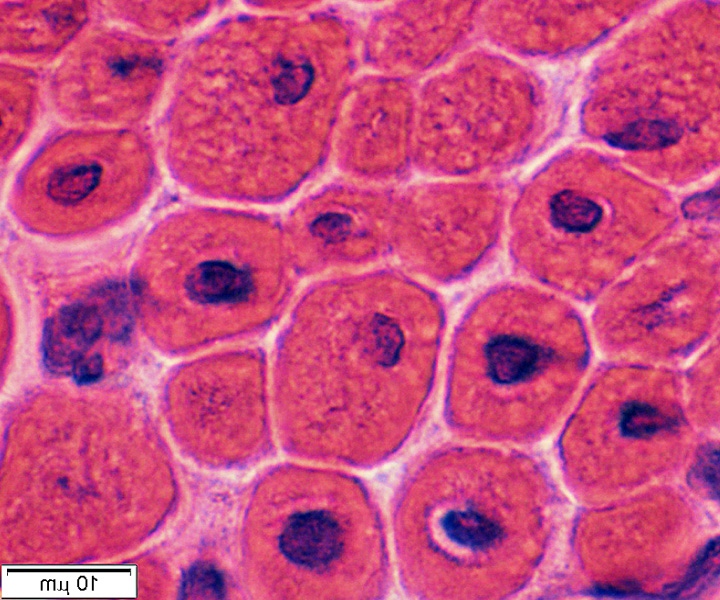 X-Linked-Myotubular-Myopathy
X-Linked-Myotubular-MyopathyX-linked myotubular myopathy (MTM) is a neuromuscular disorder caused by mutations in the
myotubularin (MTM1) gene. It is a rare condition (estimated at 1:50,000 male births) associated with substantial morbidities and early mortality.
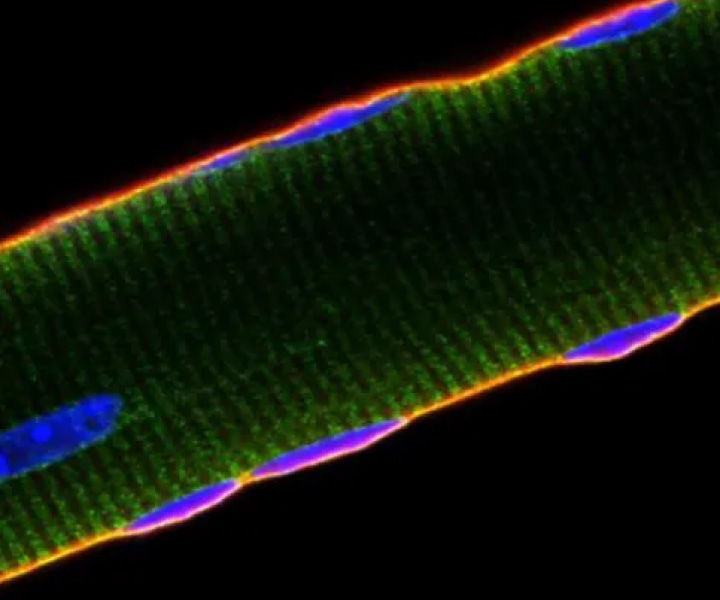 Muscle-2
Muscle-2The Myosana platform is amenable to treatment of a broad range of neuromuscular disorders and cardiomyopathies, which we will pursue after moving forward on our first target, Duchenne muscular dystrophy (DMD).